
The FDA approval is based on ‘substantial’ disease-free survival results and may ‘drastically’ improve lung cancer survival.

The FDA approval is based on ‘substantial’ disease-free survival results and may ‘drastically’ improve lung cancer survival.

"Dr. Rosenzweig’s commitment to helping patients with lung cancer and mesothelioma is truly heroic," several colleagues write.

These are the powerful stories of real-life heroes in the lung cancer space who persevere with great compassion and teach us to embrace, not avoid, life’s setbacks and challenges.

"Dr. Hossein came to the United States by himself in his teens as a political refugee from the turmoil in Iran," writes a colleague who nominated him for the Lung Cancer Heroes® Award. "Completely on his own and through his hard work, scholarship, initiative and refusal to let things get him down, he went to college and then to medical school."

CURE®’s Lung Cancer Heroes® Award Program celebrates and thanks the heroes who make a difference in the lives of patients with lung cancer.
Treatment with the immunotherapy Opdivo for relapsed, malignant mesothelioma may represent a useful choice for patients with minimal therapy options.

From former New York Islanders hockey player Mike Bossy announcing that he has lung cancer to First Lady Jill Biden emphasizing the importance of breast cancer screenings, here’s what’s happening in the cancer landscape this week.

A company focused on treating underserved patients announced that the first patient with an NTRK fusion was dosed in a phase 2 trial of taletrectinib this summer.

The Food and Drug Administration approved adjuvant Tecentriq for patients with PD-L1—positive, stage 2 to 3A non-small cell lung cancer.

Aileron Therapeutics, Inc. announced the initiation of a clinical trial for ALRN-6924, a treatment for patients with non-small cell lung cancer.
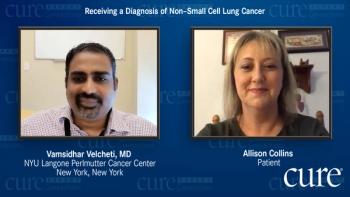
Allison Collins reflects on the genetic testing she underwent after receiving a non-small cell lung cancer diagnosis and describes the treatment options presented to her at that time.

A woman explains how she received a lung cancer diagnosis while she was caring for her sick husband and what it was like to lose him in this episode of the “CURE® Talks Cancer” podcast.

Dr. Vamsidhar Velcheti provides an expert’s insight on the testing and staging that must be performed during a diagnosis of non-small cell lung cancer.

Considerations for the emotional impact of a lung cancer diagnosis and how patients can be supported during their treatment journey.

Allison Collins describes her diagnosis with KRAS-positive non-small cell lung cancer and the symptoms that led her to seek medical attention.

Here’s what patients should know about the recent FDA approval of Exkivity, the first oral therapy for patients with non-small cell lung cancer who have EFGR exon 20 insertion mutations.
Geographic disparities at the state-level are leading to increased rates of death from lung cancer, according to an expert.

The first patient was treated in the phase 2b KICKSTART trial testing the safety and efficacy of tomivosertib in combination with Keytruda.

The prescription of systemic cancer therapies varies based on a patient’s age, comorbidities, cancer stage and other variables, according to recent research.

Ms. Addario, lung cancer survivor, encourages patients with non-small cell lung cancer to use all the resources and tools that are available to them.

Ms. Addario shares the promising hope for patients with lung cancer and describes the steps in the process of biomarker testing for patients with lung cancer.

Treating patients with lung cancer by identifying specific biomarkers to use the correct combination therapy and what it means for a biomarker to be actionable.
The average survival for patients with malignant pleural mesothelioma was less than a month after being diagnosed with COVID-19, according to a small study.

The eligible age of 65 years for Medicare may be discouraging patients from getting screenings and tests as they wait for more health coverage.

The FDA’s approval of Exkivity marks the first approved oral therapy for this subgroup of patients with non-small cell lung cancer.