Colorectal Cancer
Latest News
Video Series

Latest Videos
Shorts

Podcasts
More News

In BREAKWATER, EC plus FOLFIRI achieved a 64.4% response rate and showed a trend toward improved overall survival in patients with BRAF V600E mCRC.
Study findings show more colorectal cancer risk reduction and safety with the use of a GLP-1RA versus Aspirin.
Physical activity, especially walking, was linked to less fatigue and better quality of life for patients with colorectal cancer, particularly in early survivorship.
Patients with stage 3 colorectal cancer who tested MRD-positive with Signatera after surgery saw a meaningful survival benefit from Celebrex plus chemotherapy.

The FDA granted fast track status to muzastotug plus Keytruda for adults with microsatellite stable metastatic colorectal cancer without liver metastases.

After a surprise diagnosis at 47, salon owner Christa Messmer found strength, faith and renewed meaning in life while facing colorectal cancer.
The FDA grants fast track status to alnodesertib plus low-dose irinotecan for third-line metastatic colorectal cancer with ATM deficiency.
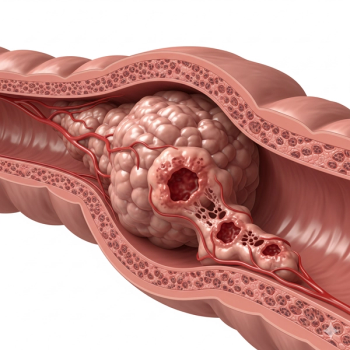
Colorectal cancer is a complex disease. Understanding its fundamental aspects can empower you to have more informed and productive conversations with your oncologist.

Rising colorectal cancer rates in younger adults are prompting earlier screenings, with colonoscopy offering both detection and prevention.

FDA grants D3S-001 treatment breakthrough therapy for KRAS G12C some with lung cancer and orphan drug status for KRAS G12C colorectal cancer in adults.
The FDA granted breakthrough device designation to Quest Diagnostics’ Haystack MRD test for patients with stage 2 colorectal cancer after surgery.

The FDA approved the Dako Omnis panel to identify patients with colorectal cancer with mismatch repair deficiency.

Dr. Zev Wainberg of UCLA recently spoke with CURE about trial results concerning the cancer vaccine ELI-002 2P.
The FDA approved an investigational new drug application for ABT-301, clearing the way for a new clinical trial in metastatic colorectal cancer to begin.

Writing helped me focus on gratitude and healing after my colon cancer diagnosis, transforming the act into a powerful recovery tool.

Three of five patients with advanced colorectal cancer responded to leronlimab, including one complete response lasting five years, according to new data.

Zanzalintinib plus Tecentriq significantly improved overall survival versus Stivarga in previously treated metastatic colorectal cancer, phase 3 data show.

Tecentriq and chemotherapy combination reduces the risk of death versus chemotherapy alone in deficient mismatch repair, stage 3 colorectal cancer.

Braftovi and Erbitux plus chemotherapy improved survival without progression over standard therapy in BRAF V600E-mutant mCRC in the first-line setting.
Tumors shrank more with the addition of acoustic cluster therapy to chemotherapy in colorectal liver metastases in the phase 1 ACTIVATE trial.

As a Lynch syndrome previvor, I feel deep gratitude to witness my son’s graduation — a milestone my brother never lived to see with his own child.

Dr. Cathy Eng discusses data that led to the FDA approval of Braftovi and Erbitux plus chemo for metastatic colorectal cancer with a BRAF V600E mutation.

Here is a recap of every FDA approval announced by the regulatory agency in the month of April, spanning various cancer types.

LUT014, a BRAF inhibitor gel, improved acne-like rashes in patients with colorectal cancer undergoing anti-EGFR therapy, according to UCLA researchers.

The Oncodetect test detects molecular residual disease across solid tumors using circulating tumor DNA to inform recurrence risk and treatment decisions.