
Here is a list of the recent trial initiations that occurred within the cancer space in December.

Here is a list of the recent trial initiations that occurred within the cancer space in December.

Real-world data shows that CAR-T therapy can lower costs for patients with pre-existing comorbidities outside of the clinical setting.
Some patients with non-Hodgkin lymphoma may soon have a treatment option that could offer complete remission, according to new data presented at the American Society of Hematology 2019 Annual Meeting.

An overview of the therapies available for patients with diffuse large B-cell lymphoma (DLBCL) and transformed follicular lymphoma.

As a plastic surgeon, patient safety is my top priority. I want anyone with breast implants to be aware of a recent recall linked to a rare lymphoma that may affect them.

Dealing with any type of illness after cancer can be a frightening experience.

In this episode of the “CURE Talks Cancer” podcast, Twist Out Cancer founder Jenna Benn Shersher shares on her journey with grey zone lymphoma and how her organization helps pair individuals to use art as an outlet to cope through cancer.
For Blood Cancer Awareness Month, here’s a round-up of the latest news and updates in this disease space.

From a freezer failure that lost the stem cells of some patients with cancer to a documentary about one of the pioneers of immunotherapy, here’s what is making headlines in the cancer space this week.

Twist Out Cancer has paired those touched by cancer with an artist, who creates a unique piece of artwork that reflects on their individual journey with cancer.

How celebrity Jon Stewart championed a cause that brought answers to my cancer diagnosis.

Treating relapsed Waldenstrom’s macroglobulinemia takes an individualized approach from a growing list of options.

Before getting breast implants, women should know about a cancer (breast implant-associated anaplastic large cell lymphoma) and an autoimmune syndrome associated with them.

Knowledge is essential for any patient facing cancer, and this issue provides the facts not only about breast implant-associated anaplastic large cell lymphoma but also other rare cancer types or mutations.

Following its announcement of covering chimeric antigen receptor-T cell therapy nationwide, the Centers for Medicare and Medicaid Services has now made this highly-priced, but life-saving, treatment available to many who would not otherwise have access to it at their cancer centers.
The first gluteal implant case has raised concern for risk of anaplastic large cell lymphoma in more than just textured breast implants.
Three-time cancer survivor and “Pink Hulk” one-woman show writer and performer, Valerie David, recalls the day she learned she had non-Hodgkin lymphoma.
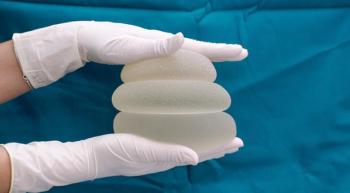
A lymphoma expert discusses implant removal, reconstruction and the signs of anaplastic large cell lymphoma.

The Food and Drug Administration has requested that the manufacturer voluntarily recall certain breast implants and tissue expanders from the market to protect women from risks such as breast implant-associated anaplastic large cell lymphoma.
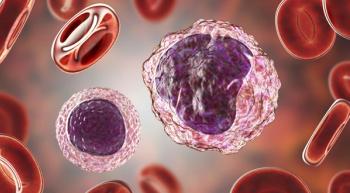
With the Food and Drug Administration’s approval of Ruxience, a biosimilar to Rituxan, patients with certain types of non-Hodgkin lymphoma and chronic lymphocytic leukemia may have improved access to a more affordable treatment option.

How an app gives a glimpse into the future for many patients with cancer and survivors who fear they won't make it to old age.

The Food and Drug Administration granted CLR 131, a targeted, molecular radiotherapy, a fast track designation.
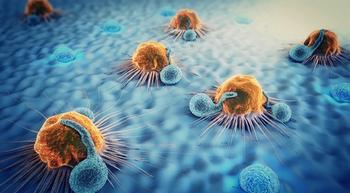
A lymphoma expert discusses the side effect profile and follow-up data from the ECHELON-1 clinical trial.

Women who undergo a mastectomy have many options and concerns to consider when undergoing breast reconstruction. My choice was simple: I wanted to look as natural as possible, but I have since learned that I have textured implants.

When I was diagnosed with Hodgkin's lymphoma, I envisioned myself in a galaxy far, far away. Just like any well-trained Jedi, I fought back when insurance denied my claim for doctor-recommended treatment.