Study results also determined that immune system status before treatment may predict outcomes post-treatment.

Study results also determined that immune system status before treatment may predict outcomes post-treatment.

In this episode of the “CURE Talks Cancer” podcast, we spoke with a teenager who was diagnosed with Hodgkin lymphoma at the start of the COVID-19 pandemic. We talked about her cancer journey, what it was like to receive treatment during the pandemic, and more.
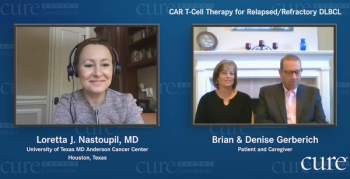
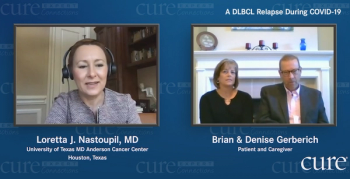
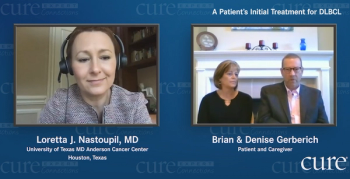
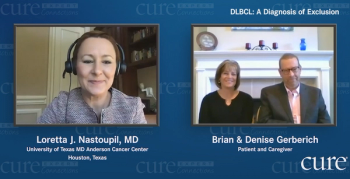

Doctors told Maryland Governor Larry Hogan to go home and rest after undergoing cancer surgery. But he had other plans: sharing his health issues.

View the entire CURE® Educated Patient® Leukemia & Lymphoma Summit. A virtual event seeking to educate, inform and challenge the thinking of patients with leukemia and lymphoma.

From acclaimed actor Jeff Bridges tweeting about a new lymphoma diagnosis to controversial conservative radio host Rush Limbaugh announcing that his stage 4 lung cancer has progressed, here’s what’s happening in the cancer landscape this week.

Data from the trial that led to the FDA approval demonstrated that Keytruda reduced the risk of progression or death in patients with relapsed or refractory classical Hodgkin lymphoma by 35%, compared to Adcetris.

In an interview with CURE®, the president of the National Center for Health Research elaborates on the effort to protect patients from potentially serious side effects associated with getting breast implants, including breast implant-associated anaplastic large cell lymphoma.
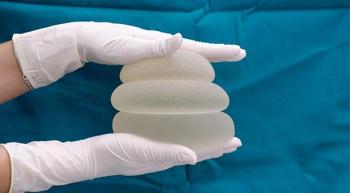
New guidance from the Food and Drug Administration recommends that the makers of breast implants include a black-box warning, a patient decision checklist and other information on product labels that will help protect them against a cancer and an autoimmune condition that can arise from use of the devices.
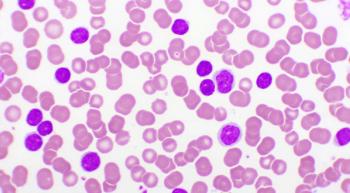
In this special hematology focused issue of CURE® we look at all the promising treatments and research for both adults and children with blood cancers.

Weighing the cost of CAR-T cell therapy in treating blood cancers is a finical burden for many patients with blood cancer.

Susan M. O’Brien, M.D., one of the nation’s foremost leukemia experts, discusses the future of frontline therapies and upcoming clinical trials associated with chronic lymphocytic leukemia.
CURE® compiled a roundup of five recent reports patients with lymphoma may have missed.

The FDA also released a video for patients that details seven things they should know about breast implants, including information about breast implant-associated lymphoma and systemic symptoms.