Leukemia
Latest News

Tibsovo Plus Vidaza Is Beneficial for Patients With IDH1-Mutant Acute Myeloid Leukemia

To My Loved Ones, ‘You’ve Held the Pause Button Long Enough’ During My Cancer Journey
Latest Videos

More News

From PGA Tour golfer Justin Thomas’ new sun-protection line to soap opera actress Marnie Schulenburg’s breast cancer death and the first child to receive CAR-T cell therapy celebrating 10 years cancer-free, here’s what’s happening in the cancer space this week.

Ilene Galinsky, ANP-BC, "had the courage to fiercely stand up for her patients’ best interests despite conventions of rank, seniority and arbitrary rules," a colleague said.
Treatment with a novel monotherapy in a patient with relapsed/refractory acute myeloid leukemia was effective enough that a successful, as indicated by a significant reduction in bone marrow blasts, stem cell transplant was performed.

On this episode of the “Cancer Horizons” podcast, a cancer nurse explains how her son’s leukemia diagnosis transformed her work, her research and her outlook on life.

Recent study results showed that the use of a remote monitoring device worn on the upper arm may help patients with blood cancers seek assistance for serious complications while receiving intensive treatments.

In this episode of the “Cancer Horizons” podcast, an expert unpacks cancer risks for individuals with Down syndrome and analyzes the results of a recent study about mortality risks for childhood survivors later in life.

Patients with Down syndrome have an increased risk for leukemia during childhood, and toxicities related to treatment may be more common in this patient population.

From a Procter & Gamble shampoo and conditioner recall due to the presence of benzene, a cancer-causing chemical, to heartwarming acts of holiday kindness for cancer survivors and their families, here’s what’s happening in the cancer landscape this week.
New findings show that 43% of patients with certain blood cancers produced COVID-19 antibodies after receiving a third full dose of the mRNA vaccine.

The Food and Drug Administration approved Orencia, which is commonly used to treat conditions like rheumatoid arthritis, to prevent acute graft-versus-host-disease in adults and children who underwent a hematopoietic stem cell transplant from an unrelated donor.
The immunotherapy Kymriah offered similar benefits in patients younger than and older than 18 years with relapsed/refractory B-cell acute lymphoblastic leukemia.

Researchers found that depression and anxiety may skew patient perceptions of participating in blood cancer clinical trials, thus contributing to low enrollment throughout the United States.

The FDA approval of Rituxan plus chemotherapy is indicated for the treatment of children aged six months to 18 years who have various forms of lymphoma or a form of leukemia.

Although the interest of at-home care including the administration of cancer infusions has grown since the start of the COVID-19 pandemic, hesitation looms over its convenience, cost and safety.

The Food and Drug Administration approved Scemblix for patients with Philadelphia chromosome-positive chronic myeloid leukemia in the chronic phase whose disease progressed on prior TKI treatment.
New research in blood cancer includes new treatment options for acute myeloid leukemia and a novel therapy using BK virus-specific T cells to treat BK virus-associated hemorrhagic cystitis.
A new T-cell therapy can be highly effective in treating BKV-associated hemorrhagic cystitis while minimizing side effects.

An expert from the Leukemia & Lymphoma Society breaks down the effectiveness of the COVID-19 vaccine in patients with blood cancers on this episode of the “Cancer Horizons” podcast.

A three-time acute lymphoblastic leukemia survivor became a bone marrow transplant nurse and now helps other patients with the same diagnosis.

Gleevec was approved by the FDA in 2001. The groundbreaking oral drug has since been a lifesaver for many patients with chronic myeloid leukemia.
Patients with blood cancers may respond differently to doses of the COVID-19 vaccines based on the treatment they’re receiving, for example.

An expert and a patient discuss their experiences with Tecartus, the newly approved CAR-T cell therapy for certain patients with acute lymphoblastic leukemia.

Natural killer cell therapy is making strides in blood cancer research, but there’s still much more to learn.
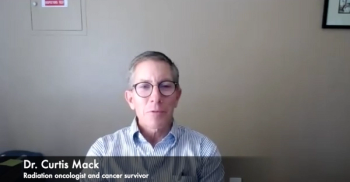
After undergoing treatment for leukemia in the middle of his career as a radiation oncologist, Dr. Curtis Mack noticed that he became a more empathetic and patient doctor.

Dr. Curtis Mack can relate to his patients on a level other radiation oncologists might not be able to because he has received a diagnosis, gone through treatment and survived cancer himself.