
Dr. Gabriela Hobbs offers insight into a rare cancer, myeloproliferative neoplasms, to help patients learn more during their treatment journey.

Kristie L. Kahl is vice president of content at MJH Life Sciences, overseeing CURE®, CancerNetwork®, the journal ONCOLOGY, Targeted Oncology, and Urology Times®. She has been with the company since November 2017.
She is a graduate of Rider University, where she acquired a Bachelors of Art in journalism, as well as a graduate of Temple University, where she received her Masters of Science in Sports Management.
Follow Kristie on Twitter at @KristieLKahl, or email her at [email protected].

Dr. Gabriela Hobbs offers insight into a rare cancer, myeloproliferative neoplasms, to help patients learn more during their treatment journey.
CURE spoke with Maimah Karmo, founder and CEO of the Tigerlily Foundation, about support after a breast cancer diagnosis.

CURE spoke with Maimah Karmo, founder and CEO of the Tigerlily Foundation, about aspects outside of breast cancer treatment, like financial toxicity, psychosocial effects and more.

CURE spoke with Maimah Karmo, founder and CEO of the Tigerlily Foundation, about disparities in breast cancer care, and how they are being addressed.

On behalf of TigerLilly Foundation, CURE spoke with Dr. Andrea Phillips about clinical trials in breast cancer.
On behalf of TigerLilly Foundation, CURE spoke with Dr. Angelique Richardson, from UCSD Health, about HER2-positive breast cancer.

In this episode of the “CURE Talks Cancer” podcast, we spoke with Dr. Laura Michaelis, a 2019 CURE MPN Hero, about how far we’ve come in the myeloproliferative neoplasm space.

In this episode of the “CURE Talks Cancer” podcast, we spoke with Rose Gerber, a breast cancer survivor and advocate, about disparities in cancer care, in particular in breast cancer.

CURE spoke with Dr. Rajiit Rampal, on behalf of the MPN Research Foundation, about MPN symptoms and their management.
CURE spoke with Brandon Goetzman, board member from the MPN Research Foundation, on elevating the patient’s voice when it comes to their care for myeloproliferative neoplasms.

CURE spoke with Dr. Gabriela Hobbs, on behalf of the MPN Research Foundation, about the need for clinical trial education for patients with myeloproliferative neoplasms.

CURE spoke with Dr. Rajiit Rampal, on behalf of the MPN Research Foundation, about disease progression in myeloproliferative neoplasms.

CURE spoke with Dr. Gabriela Hobbs, on behalf of the MPN Research Foundation, about currently available treatment options, as well as those under clinical investigation, for patients with myeloproliferative neoplasms.

In its Speaking Out video series, on behalf of Aim at Melanoma, CURE ® spoke with Dr. Sunandana Chandra about emerging therapies in melanoma.

On behalf of AiM at Melanoma, Drs. Jeffrey Farma and Sunandana Chandra discuss current and future treatment options for melanoma, including a rare subtype.
Results from a recent study strongly support a treatment regimen of Darzalex with Revlimid and dexamethasone as a new standard of care for patients with transplant-ineligible newly diagnosed multiple myeloma.

On behalf of the Head and Neck Cancer Alliance, Dr. Michael Moore spoke with CURE® about emerging therapies that potentially offer exciting new options for the future.
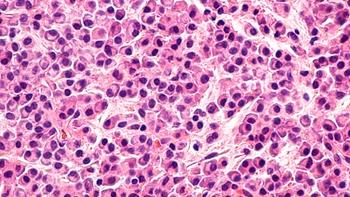
After long-term follow-up, the CAR-T cell therapy still boosted survival outcomes in patients with relapsed/refractory multiple myeloma, regardless of the number of prior lines of therapy received.
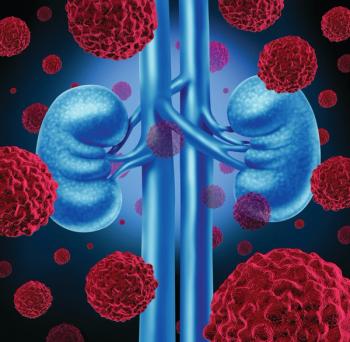
In patients with metastatic renal cell carcinoma whose disease failed to respond to two prior therapies, Fotivda, compared with Nexavar, contributed to a duration of response of 20.3 months versus 9 months.
Treatment with chemotherapy following the standard chemoradiation treatment failed to improve multiple survival outcomes in women with locally advanced cervical cancer.





Clinical trials are often the key to advancements in treating ovarian cancer. As part of its “Speaking Out” video series, CURE® spoke with Dr. Debra Richardson about what patients should know about joining a trial.

As part of the “Speaking Out” video series, CURE® spoke with Dr. Laura M. Kulik about the basics of liver cancer, highlighting the need for a multidisciplinary approach to care.

As part of its “Speaking Out” video series, CURE® spoke with Katie Brown from LUNGevity about challenges that have arisen from the COVID-19 pandemic — including delays in screening — and resources to help patients through these trying times.

In partnership with CURE®, Bonnie J. Addario and the GO2 Foundation for Lung Cancer will release “The Living Room: A Lung Cancer Community of Courage,” a collection of stories from patients, caregivers and survivors taking a personal approach to cancer care.

On Feb. 18, CURE hosted a webinar focused on educating patients on biomarker testing. Here, we highlight key takeaways from our panelists.