
Skin Cancer
Latest News
Latest Videos
More News
Active surveillance in sentinel lymph node positive melanoma could delay surgery but may result in the same risk of the cancer coming back.

Chronic immune-related adverse events from anti-PD-1 therapy occur in many patients with advanced melanoma, but shouldn’t deter them from seeking treatment that can provide long-term survival, according to an expert from Vanderbilt University Medical Center.
FDA approval of immunotherapies and BRAF/MEK inhibitors may have impacted overall survival, with a change from seven months between 2010 and 2014 to 13 months between 2015 and 2019.

As the winter months stretch on, those looking to treat Seasonal Affective Disorder (SAD) may be tempted to use indoor ultraviolet (UV) tanning devices. Not only is indoor tanning a dangerous habit, it does not successfully treat SAD.

A patient with recurrent basal cell carcinoma discusses his cancer journey, his latest challenge and how he’s now prioritized quality of life over quantity.

Intratumoral injection—the boosting of the immune response against metastatic melanoma by the direct injection of immunotherapy into tumor masses—is a promising treatment method because it’s effective AND it seems to cause fewer toxicities for the patient than combination systemic immunotherapy.

Gina Roller’s best friend, Kerri-Lynn Larimer, adopted and lived by the motto “crazy not to” long before she was diagnosed with metastatic melanoma.

We answer this question with the help of Mohammed Kashani-Sabet, M.D., the Director, Center for Melanoma Research and Treatment; and Medical Director, Cancer Center, California Pacific Medical Center.

We answer this question with the help of a pharmacist at a large oncology practice who wishes to remain anonymous.
Avastin, in combination with carboplatin plus paclitaxel (types of chemotherapy), improved progression-free survival and overall survival compared with those who received chemotherapy alone for patients with untreated advanced mucosal melanoma.
Combination treatment with Opdivo and Yervoy may have better overall survival rates for patients with metastatic uveal melanoma.

In this episode of the “CURE Talks Cancer” podcast, we spoke with someone who has undergone 41 surgeries and physically debilitating treatments during his more than 15-year journey with several cases of skin cancer. We discussed his cancer journey, and why, as he starts a new treatment after his most prior therapy failed, he’s focused on quality rather than quantity of life.
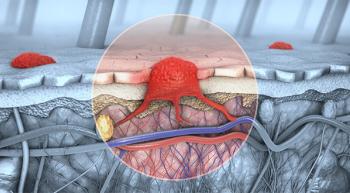
A roundup of some skin cancer news and updates that occurred in 2020 that patients may have missed.

Machine learning may be used to predict disease progression in patients with advanced melanoma.

Patients with resected, high-risk, stage 3 melanoma who were randomized to receive Keytruda (pembrolizumab) achieved a three-year recurrence-free survival rate of 63.7% compared to 44.1% in patients randomized to placebo.

A two-time cancer survivor looks back at her cancer journey and offers 4 tips for a first-time patient with cancer.
Taking cholesterol-lowering drugs may provide a mortality benefit in women with newly diagnosed colorectal cancer, breast cancer and melanoma, although more research is needed to confirm this potential association.

Recent immunotherapy approvals for patients with metastatic melanoma has widened the treatment landscape, but new research shows that patients associated with positive sociodemographic factors are more likely to receive immunotherapy.
CURE® compiled a roundup of five recent reports patients with skin cancer may have missed.

In this episode of the “CURE Talks Cancer” podcast, we spoke with Dr. Jeremy Brauer, on behalf of the Skin Cancer Foundation, about current treatment strategies and the future of the disease.

From Washington Football Team head coach Ron Rivera announcing he has cancer but plans to continue coaching this season, to ”Dancing With the Stars” judge Len Goodman revealing he had surgery to remove skin cancer from his face, here’s what’s happening in the cancer landscape this week.
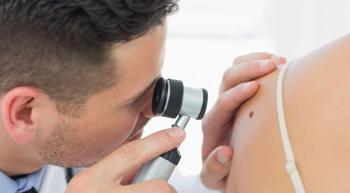
“Through patients’ eyes, augmented intelligence may improve health care quality but should be implemented in a manner that preserves the integrity of the human physician-patient relationship,” the authors wrote.
“With data showing a two-fold increase in disease-free survival with the vaccine alone and in combination with checkpoint inhibitors, we hope to one day change the narrative for people with melanoma — turning this disease into a chronic condition that can be treated and managed over time,” said Dr. Mark B. Faries.

From the recent Beirut explosion compromising pediatric oncology hospitals, to researchers looking at how intestinal bacteria can supercharge targeted immunotherapies, here’s what’s happening in the cancer landscape this week.

The Food and Drug Administration has approved Tecentriq in combination with Cotellic and Zelboraf for the treatment of patients with BRAF V600 mutation-positive advanced melanoma.






