
Dr. Scott T. Tagawa discusses what patients with prostate cancer should know about the potential benefits and risks of participating in a clinical trial.

Dr. Scott T. Tagawa discusses what patients with prostate cancer should know about the potential benefits and risks of participating in a clinical trial.

Most patients in accredited hospitals had smoking discussions documented, showing key care opportunities and smoking’s impact on treatment outcomes.

After testicular cancer, I’ve learned to guard my mood, seek small joys and embrace healing and renewal with every sunrise this spring.
The first patient with Cyclin E1+ platinum-resistant ovarian cancer has been dosed with azenosertib in part 2a of the phase 2 DENALI clinical trial.

New results from a breast cancer-related lymphedema study show the importance of measuring fluid and body composition before treatment begins.
Enrollment and initial dosing of PAS-004 at 30 milligrams has begun in three patients with MAPK pathway-driven advanced solid tumors.

Here is a recap of every FDA approval announced by the regulatory agency in the month of April, spanning various cancer types.

Breast cancer treatment is a physical, emotional, and mental challenge. Facing cancer and its side effects demands extraordinary physical, emotional, and spiritual stamina.

Dr. Neha Mehta-Shah discusses ways patients with rare blood cancers, such as lymphoma, can better understand their specific subtype of disease.

Triage Health offers free, expert-led education on the legal and practical issues that impact individuals coping with chronic or serious medical conditions.

Pancreatic cancer is one of the most challenging cancers to treat.
A new endoscopic tunneling method, STER, allowed a patient to have a GIST removed without surgery, allowing quicker recovery and fewer complications.

Teddy bears offer emotional comfort and connection for adults with cancer, easing anxiety and fostering trust during treatment.
A patient with heavily pretreated relapsed/refractory diffuse large B-cell lymphoma achieved complete remission following novel in vivo CD19 CAR-T therapy.

A phase 1 trial has initiated and is investigating treatment with ziftomenib plus Gleevec in advanced GIST following progression on Gleevec.
LUT014, a BRAF inhibitor gel, improved acne-like rashes in patients with colorectal cancer undergoing anti-EGFR therapy, according to UCLA researchers.

A breast cancer diagnosis brings about many losses.

The FDA has completed its 30-day review of an application for Hepzato in combination with standard of care in liver-dominant metastatic breast cancer.

Patient and disease-related factors influence surgical recovery and treatment outcomes after Keytruda and Padcev in advanced urothelial cancer.

Aidixi plus BCG showed strong early efficacy and manageable safety in patients with high-risk, HER2-expressing non–muscle-invasive bladder cancer.

Four years after remission from follicular lymphoma, ongoing fear of relapse, lingering symptoms, and life stressors continue to weigh heavily on me.
CM24 plus Opdivo and chemo improved survival in pancreatic cancer with CEACAM1 biomarkers, supporting a biomarker-driven phase 2b study.
Investigators shared real-world data showing a clear and consistent association between increased tumor size and mortality for patients with brain cancer.
A phase 1 trial evaluating SENTI-202 demonstrated positive preliminary results in treating relapsed/refractory acute myeloid leukemia.
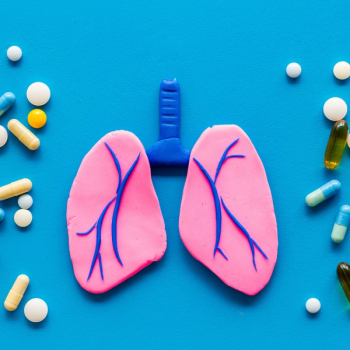
The Ventana TROP2 RxDx Device, a computational companion diagnostic, was granted breakthrough device designation by the FDA in non–small cell lung cancer.

Dr. Hyung L. Kim discusses how the addition of cytoreductive nephrectomy to ICI therapy may affect survival and quality of life those with kidney cancer.

TAR-200 showed high complete response rates in BCG-unresponsive, high-risk non-muscle invasive bladder cancer with CIS in the phase 2b SunRISe-1 study.

A chance meeting with my childhood friend reminded me — and others — that living with cancer means taking things one day at a time.

The FDA has granted approval to treatment with Tepylute, a ready-to-dilute version of thiotepa, at 100 mg for breast and ovarian cancers.

INX-315, a CDK2 inhibitor, received FDA fast track designation for patients with CCNE1-amplified platinum-resistant ovarian cancer.