
What to do when cancer isn't ruining your summer...

A new book delves into the stories of 25 immunotherapy pioneers and the history of the field they helped to develop.

Part three in a three part series about my trip to Chicago for HealtheVoices 2018.

This year’s survey is expanded to better learn about the experience of caregivers, with a selection of questions specifically for them. The Alliance asks that just one caregiver per patient fill out the survey.
Immunotherapy is being tested in the second-line setting for metastatic bladder cancer. Pending trial results, the role of chemotherapy for this patient population may change.

The American Society of Clinical Oncology issued guidelines in 2017 to support the use of meditation, yoga, acupuncture and music therapy among to help relieve side effects from cancer treatment.
CURE spoke with the chief medical officer of the Leukemia & Lymphoma Society to hear what she thinks are important next steps in improving patient outcomes.
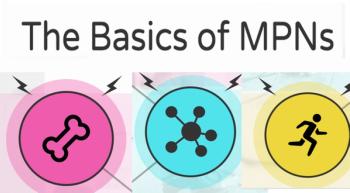
An MPN diagnosis can be overwhelming, but here are some of the basic facts every newly diagnosed patient should know.

Immunotherapy’s effectiveness may depend on the bacteria living in patients’ gastrointestinal tracts, studies show.

Novel immunotherapies either release the immune system’s parking brake or hit its gas pedal.

According to findings from the ASCO Annual Meeting, insurance disparities still exist, and may even contribute to cancer-specific and comorbidity-associated mortalities in patients with early-stage non-small cell lung cancer.
Phase 2 study results showed that patients with metastatic or unresectable, FGFR-positive bladder cancer may have a third-line option for treatment.

Sometimes the people who inspire us are closer than we realize.

The FDA has incorporated PD-L1 status into the labels for Keytruda (pembrolizumab) and Tecentriq (atezolizumab) for existing frontline approvals for platinum-ineligible patients with urothelial carcinoma, based on lower overall survival (OS) rates with the PD-1/PD-L1 inhibitors compared with platinum-based chemotherapy for patients with PD-L1–low expressing platinum-eligible urothelial carcinoma.

MMRF Honored for Fifteenth Year for Outstanding Stewardship of Donors’ Funds
Treatment with chimeric antigen receptor (CAR)-T cell therapy showed encouraging rates of durable responses among adult patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL), according to updated data from the pivotal JULIET trial.

A cancer diagnosis means lots of treatment appointments, decisions to make and fears to face. Setting goals, whether short-term or long-term, can help get you through the journey.

Part two in a three-part series about my trip to Chicago for HealtheVoices 2018.

Breast cancer survivor contemplates her breast reconstruction choices.

Cancer terminology isn't always easy to understand but in this post, hopefully you'll understand two of them much better.


Why the proverb, "you should never judge a man until you've walked a mile in his shoes" is true for both cancer patients and survivors.

Dewayne Johnson is the first of hundreds of patients to take Monsanto to trial, claiming that Roundup caused them to get cancer. His case will be seen even sooner than others because in California dying plaintiffs can be given expedited trials.

A cancer survivor discusses balance, courage and confidence.

I’m writing to say how much I missed you this Father’s Day. It’s hard to believe that it’s been eight years since I told you how much you mean to me in person. And to think you were so worried about my breast cancer recurring; the thought that your only child and mother of your granddaughters might suffer a long and painful death – as did your own mother – before you died yourself.

Researchers identified six gene variations that play an important role in the treatment and surveillance of children with medulloblastoma, for which they developed screening and counseling recommendations.

Part one in a three part series about my trip to Chicago for HealtheVoices 2018

While getting old and being ill can both cause a person to slow down, they are not the same thing.

This two-time cancer survivor is worn out, but not quitting.

For many years, breast cancer survivors have focused their hope on the number five. With the help of their oncologists, they've taken a big sip of this medical Kool aid. But are we hoping in an untruth? This survivor shares her perspective.