
Patients whose disease contained the osteopontin protein tended to have more aggressive liver cancer that did not respond to treatment.

Brielle Benyon, Assistant Managing Editor for CURE®, has been with MJH Life Sciences since 2016. She has served as an editor on both CURE and its sister publication, Oncology Nursing News. Brielle is a graduate from The College of New Jersey. Outside of work, she enjoys spending time with family and friends, CrossFit and wishing she had the grace and confidence of her toddler-aged daughter.
Follow Brielle on Twitter @Brielle_Benyon.

Patients whose disease contained the osteopontin protein tended to have more aggressive liver cancer that did not respond to treatment.

Do you keep your job? Your friends? One cancer survivor discusses her decisions after being told her non-Hodgkin lymphoma was in remission.

Approximately 18 months after granting Padcev an accelerated approval, the Food and Drug Administration granted a regular approval to the treatment for certain previously treated patients with locally advanced or metastatic urothelial carcinoma.

Most people surveyed knew about the link between alcohol and liver cancer, but far fewer knew that drinking could increase breast cancer risk, too.

Pap tests should be replaced by more accurate HPV DNA tests, the organization says.

The Food and Drug Administration (FDA) approved Keytruda (pembrolizumab) for the treatment of patients with locally advanced cutaneous squamous cell carcinoma (cSCC) that cannot be cured by surgery or radiation.

Will patients be given a placebo? Are clinical trials safe? An expert answers these questions and more.
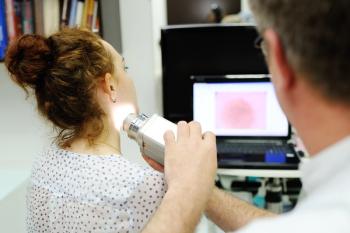
Patients with basal cell or cutaneous squamous cell carcinoma tend to have good outcomes when the disease is caught early. For those in later stages, new treatments bring hope and better prognoses.

Recent research found that women with a history of pregnancy – even if it was not carried to term – had a decreased risk of ovarian cancer.

Dave Fleischer’s 15,000-mile journey turned out to be more impactful than he anticipated. He traveled nationwide to spread awareness about cholangiocarcinoma, genetic testing and getting second opinions.

Recent research found that breast implants with a specific texture tended to result in a lower level of inflammation and immune reactions than other implants.
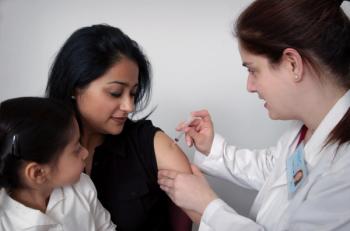
From the rapid vaccine development to heart risks and delaying immunization, we spoke with an infectious disease expert on common worries people have about getting the COVID-19 vaccine.

The disease, which advanced to his spine and bones, prevented him from appearing onstage for the “Friends” reunion.
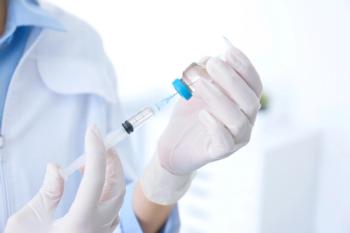
Treatment with an investigational drug induced high response rates in patients with relapsed or refractory multiple myeloma.
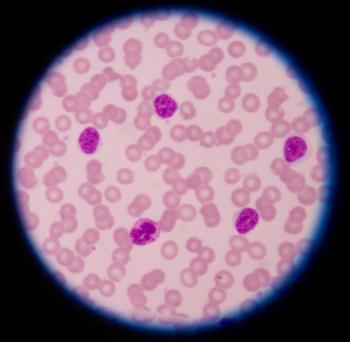
Although survival rates were similar, patients with chronic lymphocytic leukemia treated with Calquence experienced fewer side effects than those who received Imbruvica.
Adding Opdivo to treatment with Yervoy or chemotherapy improved survival, compared with chemotherapy alone in patients with unresectable and advanced esophageal cancer.

Early findings show that glofitamab may be a safe and effective option for patients with pretreated, relapsed/refractory B-cell non-Hodgkin lymphoma.

Progress on palliative care education and adoption in cancer centers has been slow, but an expert from The University of Texas MD Anderson Cancer Center says studies show that it can improve cancer outcomes.

An expert from the Massachusetts General Hospital Cancer Center recently spoke with CURE® and discussed how mindfulness can benefit cancer survivors.

Mindfulness practices have been associated with improved mental health outcomes in patients with cancer. Here, an expert shares tips on how patients can strengthen their “mindfulness muscle.”

Last week, the Food and Drug Administration (FDA) was scheduled to make a decision on whether or not they would approve oral paclitaxel plus encequidar for the treatment of metastatic breast cancer. The agency decided not to approve the regimen, and issued a complete response letter outlining why.

Merck – the manufacturer of Keytruda (pembrolizumab) – is pulling the immunotherapy drug’s indication for patients with metastatic small cell lung cancer whose disease progressed on or after platinum-based chemotherapy and one or more prior line of therapy.

Women with a history of depression had higher rates of cancer-related fatigue, according to recent study results.

There are certain patient characteristics that are associated with fear of recurrence. However, there are simple steps that survivors can take to cope.
While aspirin has been shown to be a protective factor against colorectal cancer in younger adults, recent findings show that the benefit is nonexistent in individuals over the age of 70.

Survivors of testicular cancer sought out mental health services more often than the general population, according to recent research.

Recent study results demonstrated that an at-home prehabilitation program may improve surgical outcomes in patients with lung cancer.
Some patients receiving opioids to treat cancer-related pain may be more susceptible than others to engage in non-medical opioid use.

Regular aspirin use may increase survival in patients with bladder and breast cancer.
The National Comprehensive Cancer Network (NCCN) recommends that patients receiving active cancer treatment get the COVID-19 vaccine.