
From a Hallmark Channel actor revealing a kidney cancer diagnosis to a donor match first-time meeting 20 years later, here’s what is making headlines in the cancer space this week.

From a Hallmark Channel actor revealing a kidney cancer diagnosis to a donor match first-time meeting 20 years later, here’s what is making headlines in the cancer space this week.

Here’s a sneak peek at what’s inside our Canadian issue.

A team of researchers examine funding among different cancer types, exposing disparities in cervical, colon, endometrial, liver and bile duct, lung, ovarian and pancreatic cancer.
BTK inhibitors and CAR-T cell therapies for mantle cell lymphoma help to expand the treatment landscape for patients.
Newer treatments for multiple myeloma come with an increased risk for thrombosis, but researchers discovered five factors that could help prevent the side effect.

Here’s a look at what’s inside our 2019 Rare Cancers special issue.

From patients and survivors taking on Washington to the MLB going gold, here’s what is making headlines in the cancer space this week.

After a 10-year battle with cancer, the beloved actress, wife, mother and advocate passed away at 80.

From a return to work for Alex Trebek to a personalized Backstreet Boys concert, here’s what is making headlines in the cancer space this week.

Here’s a sneak peek at what’s inside our Summer 2019 issue.

A nearly 10-year stomach cancer survivor works with Debbie’s Dream Foundation to raise disease awareness and raise money for a cure.

From an FDA warning to real-life superheroes, here’s what is making headlines in the cancer space this week.

The Food and Drug Administration approved Rozlytrek (entrectinib) for adult and adolescent patients 12 years of age and older whose cancers have the NTRK gene fusion and for adults with non-small cell lung cancer whose tumors are ROS1-positive and have spread to other parts of the body.

Here’s a sneak peak at what is inside Heal’s Summer 2019 issue.

Katie Couric reflects on life and lessons learned after losing her husband to cancer.

Poison’s Rikki Rockett discusses the immunotherapy clinical trial that led him into remission.
The first gluteal implant case has raised concern for risk of anaplastic large cell lymphoma in more than just textured breast implants.
Immunotherapies and targeted therapies may cause skin, nail, hair and foot reactions that could reduce patient quality of life.
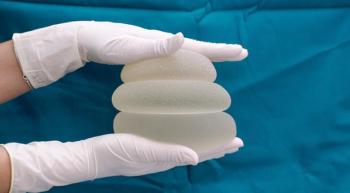
A lymphoma expert discusses implant removal, reconstruction and the signs of anaplastic large cell lymphoma.
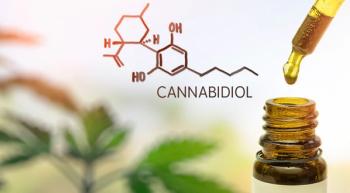
Curaleaf, a Massachusetts-based marijuana producer and retailer, is accused of more than a dozen unfounded claims regarding its products containing cannabidiol.
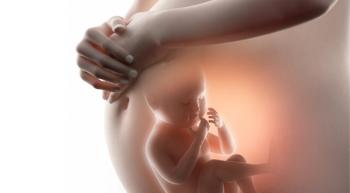
Children born to women who are considered severely obese have greater chances of developing cancer before the age of 5, according to new study findings.

Researchers examined what goes into treatment decision-making for patients with multiple myeloma.

Researchers worldwide believe low-dose light therapy may replace opioids in treating oral mucositis, a common side effect of certain cancer treatments.
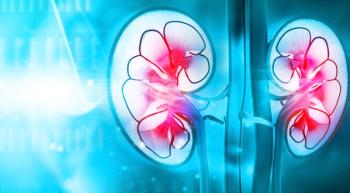
Patients with renal cell carcinoma who were treated with a targeted therapy lived three months longer than people who received older treatments.
A medical oncologist discusses the findings of three clinical trials presented during the 2019 American Society of Clinical Oncology Annual Meeting.

The Food and Drug Administration granted CLR 131, a targeted, molecular radiotherapy, a fast track designation.
A lymphoma expert discusses the side effect profile and follow-up data from the ECHELON-1 clinical trial.

Doing a self-assessment, identifying opportunities/obstacles and creating an action plan will help cancer survivors to transition into a new profession, according to one career coach.

Asking an employer to modify a work space or schedule may help patients adjust to returning to work during cancer treatment.
Newer treatments for MCL offer hope to patients living with the fast-growing and challenging-to-treat disease.