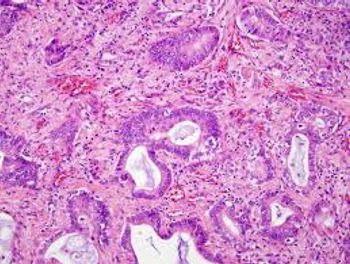
The use of the chemotherapy regimen FOLFOXIRI plus Avastin has increased from 2013 to 2022 in patients with metastatic colorectal cancer, particularly in patients aged 50 years and younger.

Darlene Dobkowski, Managing Editor for CURE® magazine, has been with the team since October 2020 and has covered health care in other specialties before joining MJH Life Sciences. She graduated from Emerson College with a Master’s degree in print and multimedia journalism. In her free time, she enjoys buying stuff she doesn’t need from flea markets, taking her dog everywhere and scoffing at decaf.

The use of the chemotherapy regimen FOLFOXIRI plus Avastin has increased from 2013 to 2022 in patients with metastatic colorectal cancer, particularly in patients aged 50 years and younger.
The top stories on chronic lymphocytic leukemia from 2023 included topics like BTK inhibitors, minimal residual disease status and approaches to care for various stages of the disease.

The pharmaceutical company submitted applications for the FDA to consider approval of Rybrevant plus lazertinib for the first-line treatment of patients with locally advanced or metastatic non-small cell lung cancer with EGFR exon 19 deletions or L858 substitution mutations.

Diet and exercise can benefit patients with cancer through side effect management and improved outcomes. CURE provides an overview of the most-read diet and exercise stories of 2023.

The FDA granted priority review of patritumab deruxtecan for the treatment of locally advanced or metastatic EGFR-mutated non-small cell lung cancer previously treated with two or more systemic therapies.
CURE shares the top stories in breast cancer, which focus on topics like treatment decisions, feelings on awareness months and books focused on breast reconstruction.

An expert explained what ROS1-positive non-small cell lung cancer is and why it is important to identify mutations like ROS1 through genetic sequencing.

An expert discussed the importance of knowing the questions to ask a patient’s care team to navigate one’s journey, including treatment and next steps.

An expert explained how ableism is present in cancer care, including chemotherapy eligibility, and how more awareness is needed in this space.

The three-year final analysis of efficacy and safety of the REACH3 trial showed that patients with steroid-refractory or dependent chronic graft-versus-host disease benefited more with Jakafi compared with the best available treatment.
Autologous hematopoietic cell transplantation, compared with CAR-T cell therapy, resulted in lower rates of relapse and improved progression-free survival in patients with relapsed large B-cell lymphoma while they were in complete response.
The quality-of-life improvement with Abecma in patients with relapsed/refractory multiple myeloma was greater when compared with standard regimens.
The recent FDA approval of the next-generation tyrosine kinase inhibitor Augtyro provides patients with locally advanced or metastatic ROS1-positive non-small cell lung cancer another treatment option when the disease becomes resistant to other therapies.
If metastatic breast cancer no longer responds to a given therapy, finding out what targetable mutations a patient has may guide the next steps for treatment, an expert said.

The FDA recently approved Tibsovo for relapsed/refractory MDS with an IDH1 mutation, a treatment option for a disease subset with no options before this approval.

In conjunction with ZERO — The End of Prostate Cancer, CURE® recently hosted the “Educated Patient® Webinar: Advancing Equity in Prostate Cancer Care.”
Patients with advanced clear cell renal cell carcinoma treated with zanzalintinib had experienced promising antitumor activity with manageable toxicity regardless of prior treatment with cabozantinib.
Circulating tumor DNA — which is observed via blood test — may be helpful in determining which patients with kidney cancer need more intense treatment and who can have their therapies deescalated.

While translocation renal cell carcinoma tends to respond well to immunotherapy, there is still a need for more investigation into targeted therapies for this patient population.
An expert explained the different treatment options for patients with estrogen receptor-positive, HER2-negative breast cancer that has recently been diagnosed or has recurred.

Managing side effects from lung cancer treatment with dose reductions is an approach experts have been using more, even though some patients voice concerns about lowering a drug’s impact on the disease.
An expert explained how next-generation sequencing can help patients with metastatic breast cancer understand their disease and available treatment options.
There are three types of myeloproliferative neoplasms, all of which come with their own set of symptoms and management strategies.
Treating patients with Tagrisso, a tyrosine kinase inhibitor, plus chemotherapy reduced the progression risk of disease of the central nervous system in patients with EGFR-positive non-small cell lung cancer.

This expanded indication broadens the use of Rozlytrek for solid tumors to include children one month old, with the original approval in 2019 only including children one year and older.
Patients with previously treated small cell lung cancer who received a 10-mg dose of tarlatamab had improved responses with no new safety signals.
One expert explained how patients with breast cancer have an increased risk for blood clots, even though one recently published survey determined that not many patients are aware of this.

The FDA approved Opdivo for the treatment of patients aged 12 years and older with stage 2B or 2C melanoma that has been surgically removed
Adding TPST-1120 to the standard of care for the treatment of unresectable or metastatic hepatocellular carcinoma, a type of liver cancer, improved progression-free survival and overall survival in a phase 1b/2 clinical study.
After discussions with the FDA, Takeda plans on voluntarily withdrawing Exkivity as a treatment for adults with EGFR exon 20 insertion mutation-positive, locally advanced or metastatic non-small cell lung cancer.