Results from an ongoing phase 2 study show the viability for the use of Brukinsa after patients with previously treated B-cell malignancies are deemed intolerant to the next-generation Bruton tyrosine kinase inhibitor Calquence.

Results from an ongoing phase 2 study show the viability for the use of Brukinsa after patients with previously treated B-cell malignancies are deemed intolerant to the next-generation Bruton tyrosine kinase inhibitor Calquence.
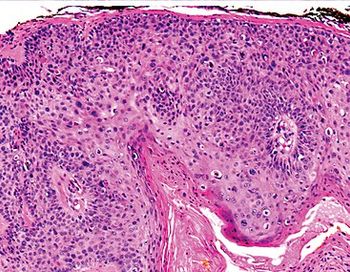
A recent phase 2 study showed promising survival improvements with neoadjuvant Libtayo in patients with cutaneous squamous cell carcinoma after a year and a half follow up.
Imfinzi plus tremelimumab led to better overall survival outcomes compared to standard chemotherapy in patients with metastatic non-small cell lung cancer.

Treatment modifications in the pre-surgery setting led to a lower rate of pathologic complete response in Black patients with breast cancer, the researchers found.

New study results demonstrate that patients with lung cancer have experienced more worries and general distress during the COVID-19 pandemic compared to other patients with cancer.
Onivyde may offer patients with small cell lung cancer whose disease has progressed after initial chemotherapy a new treatment option, according to updated study results.

From a urologist with a terminal cancer diagnosis receiving tickets to the 2021 Super Bowl to see his favorite quarterback Tom Brady compete for a seventh championship, to Indiana Pacers guard Caris LeVert undergoing surgery for renal cell carcinoma, here’s what’s happening in the cancer landscape this week.

Biomarkers play an important role in treatment decisions of many tumors, but in lung cancer they are a deciding factor between which immunotherapy is the best option.

After a fast-track designation, the Food and Drug Administration approved the combination of Cabometyx and Opdivo in the firstline setting for patients with advanced or metastatic renal cell carcinoma.

From a Marine veteran walking 8,500 miles over the last 24 years to raise cancer awareness and funds for treatment to researchers using cancer cells from a deceased osteosarcoma donor to help advance treatment, here’s what’s happening in the cancer landscape this week.

The researchers concluded that the overall low health-related quality of life scores for young CRC survivors in the categories of social and functional well-being should be targeted by appropriate methods, which could include counseling and quality of life interventions.

From an oncologist forgiving the medical debt of 200 of his patients to “Saved by the Bell” Star Dustin Diamond being diagnosed with stage 4 cancer, here’s what’s happening in the cancer landscape this week.

In an interview with CURE®, lead study investigator of the Keynote 355 trial, Dr. Hope S Rugo, discusses the results of the study that led to the FDA approval of the combination therapy and what further data means for certain patients with metastatic triple-negative breast cancer.

From one leukemia survivor organizing essential blood drives for patients with cancer as COVID-19 delays hit, to a kindergarten teacher handling a recurrence of ovarian cancer by continuing to teach her students through Zoom, here’s what’s happening in the cancer landscape this week.

From the Ohio state legislature tackling the “fail first” provision insurances put in place to keep patients with advanced cancer on generic drugs that are less effective to COVID-19 becoming the third leading cause of death in the United States behind cancer, here’s what happened in the cancer landscape this week.

From a new study showing data that patients with cancer, especially Black patients with cancer, are at a higher risk for COVID-19 than non-cancer patients to NFL punter Rigoberto Sanchez making a speedy recovery after surgery for cancer, here’s what’s happening in the cancer space this week.

For multiple patients with blood cancers who would normally use Rituxan, the Food and Drug Administration has approved the biosimilar Riabni based off highly similar clinical evidence between the two drugs, potentially lowering costs for patients.
The complexity of multiple myeloma can make it difficult to treat, but with complexity comes more avenues for researchers to explore what treatments work best. Recently, CURE® spoke with an expert from Dana-Farber Cancer Institute on what patients can expect to see from the clinical setting.

The Food and Drug Administration has issued the first emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 in people 16 years old and older.
An increase in gastrointestinal side effects were found with the more effective combination of oral paclitaxel and encequidar; however, new safety evaluations demonstrated that various antiemetics can help treat these effects in patients with metastatic breast cancer.

From actor Jason Momoa gifting a trident to a young fan of the superhero movie “Aquaman” who is receiving treatment for an aggressive form of brain cancer, to data demonstrating the regular consumption of chili peppers is associated with lower cancer mortality rates, here’s everything happening in the cancer landscape this week.
The early study of the investigational CAR-T cell therapy lisocabtagene maraleucel shows how patients with relapsed/refractory CLL/SLL may have a viable new treatment option.

In an interview with CURE®, a Waldenstrom’s macroglobulinemia expert discusses the long-term benefits of the targeted combination of Imbruvica and Rituxan.

Imbruvica treatment in patients with chronic lymphocytic leukemia whose disease expressed a certain mutation elicited sustained efficacy over a median follow-up of four years, according to data from a long-term analysis.

In an interview with CURE®, Dr. Jan A. Burger discusses how the results of two phase 3 studies could help redefine what constitutes as low or high risk in patients with CLL or SLL.

In an interview with CURE®, lead study investigator Dr. John Mascarenhas discusses what the results of the phase 2 IMbark study could mean for the future of patients with myelofibrosis.

From a recently published study showing preliminary connections between elevated levels of stress-related hormones and cancer recurrence, to a Texas mayor receiving a diagnosis of cancer and COVID-19, here’s what’s happening in the cancer landscape this week.

From the National Cancer Institute releasing promising research data into the genomic mutations that helped “exceptional responders” survive on treatment when others didn’t, to a 15-year-old Kentucky cancer survivor dying from COVID-19, here’s what’s happening in the cancer landscape this week.

From actress Mindy Kaling announcing a partnership with PanCaN to raise awareness for pancreatic cancer after losing her mother to the disease, to Al Roker sharing successful prostate cancer surgery news, here’s what’s happening in the cancer landscape this week.

The Food and Drug Administration has approved the combination of Keytruda (pembrolizumab) with chemotherapy for patients with locally recurrent unresectable or metastatic triple-negative breast cancer who have a PD-L1 expression.

Published: January 15th 2021 | Updated:

Published: September 4th 2020 | Updated:

Published: December 7th 2020 | Updated:

Published: December 6th 2020 | Updated:

Published: October 23rd 2020 | Updated: