
Learning to accept the physical changes breast cancer creates is often difficult, but doable. It takes time to learn to embrace change but doing so is beneficial to well being.

Learning to accept the physical changes breast cancer creates is often difficult, but doable. It takes time to learn to embrace change but doing so is beneficial to well being.

During cancer treatment, patients lose a lot of things, but one thing that’s constantly noticed is hair loss.
Treatment options for patients with HER2-positive breast cancer continue to expand, especially after the Food and Drug Administration (FDA) approval of Nerlynx (neratinib) earlier in 2017 and the more recent priority review designation of Perjeta (pertuzumab).

After tracking the progress of about 20 patients with brain cancer who used cannabis, a neuro-oncologist found that the substance caused them no harm, had few side effects and did not interfere with conventional treatments.

To offset an increased risk for serious side effects, patients with chronic lymphocytic leukemia say they prefer a substantial increase in progression-free survival in order to accept this negative treatment attribute.
A researcher discusses the exciting times surrounding the development of novel agents designed to treat multiple myeloma.
Imagine being diagnosed at the age of 21 as a never-smoking individual and college athlete.

Breast cancer survivor shares her journey after testing positive for a newly discovered genetic mutation (PALB2) related to breast cancer.

Treatment for metastatic cancer can mean asking a tough question every single day.

Taking time to show gratitude for compassionate care is important. It can be done any time of year but the holiday season offers a perfect opportunity.

Cancer treatment isn't what it used to be, and it is important that patients and their loved ones understand that.

This cancer survivor learned how mindfulness can change the way we heal.

Do a few weeks delay in surgery really impact outcomes for patients with colon cancer?
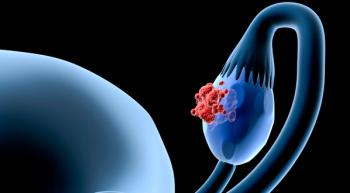
As PARP inhibitors continue to improve outcomes in patients with ovarian cancer, they may one day be moved into the frontline treatment setting, said Susana M. Campos, M.D., a gynecologic oncologist at Dana-Farber Cancer Institute and an assistant professor at Harvard Medical School.

There are plenty of things to talk about that can keep your mind from the fears and concerns that hover around cancer.

Lymphedema is a common side effect that may affect a cancer survivor months or even years after treatments such as surgery or radiation therapy. Here are some tips to help manage lymphedema.

Cancer survivor and motivational clutter-clearing trainer offers ideas to clear the clutter and the stress from your holidays.

How to survive your own breast cancer survival surprise party.

The disease is both physically and emotionally taxing. Now, a new study finds that more than 1 in 5 people diagnosed with cancer suffer from PTSD. Some even experience symptoms years later.

The holidays can be wonderful and tough at the same time. Your approach to them is what makes all the difference.

November is the month of gratitude. Something I take as a gift from cancer is trying to stay connected with my ability to have gratitude and to connect with kindness.

Singing during or after cancer treatment may be very beneficial.

I know I’ll never be Thor, but I do know the best way to ingest fungus.
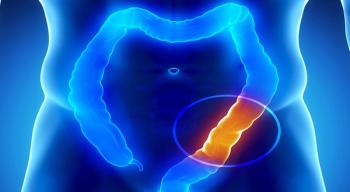
“The very exciting field of checkpoint inhibitors is still evolving, and we are trying to understand where they fit in the world of CRC therapy,” said McCollum, a hematologist and medical oncologist at Texas Oncology.

Everybody copes differently with cancer, even within a family unit.
The combination delated progression or death compared to Avastin and chemotherapy alone, according to Roche, the manufacturer of the anti–PD-L1 and anti–VEGF agents.

Silicone doesn't last forever. After a few years of constant wear, a prosthesis breaks down and is no longer functional. Some women become very attached to their replacement breasts and find it difficult to say goodbye. This is one survivor's light hearted look at what to do with worn out breast forms.

Not for the faint of heart, cancer challenges every aspect of our lives.

When consulting with her team privately, I did my best to maintain my composure. It was not lost on me that we were in that room because my older sister had cancer. And often, we were more specifically in that room because she was not responding to treatments.