
Through fanciful underwater photos of people with metastatic breast cancer, the Serenity Project aims to raise awareness and funds.

Through fanciful underwater photos of people with metastatic breast cancer, the Serenity Project aims to raise awareness and funds.
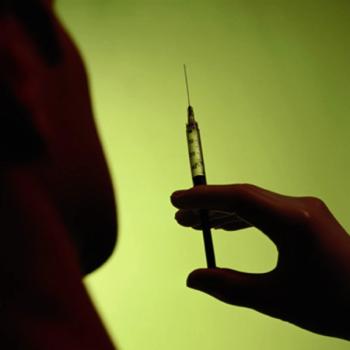
The designation recognizes that the vaccine, an immunotherapy called SurVaxM, would treat a rare disease, defined as one that affects a patient population of less than 200,000 Americans. However, because it acts on a protein that appears in 80 percent of cancers generally, SurVaxM may have wider applications.
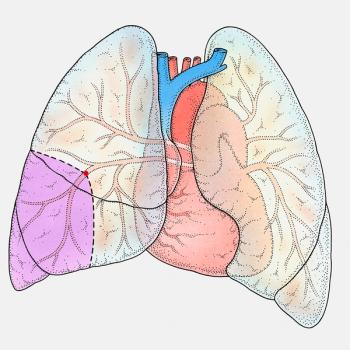
When patients are diagnosed with lung cancer, one of the first questions they're often asked is, "Do you have a history of smoking?"

Bills mysteriously paid. Old friends reunited. These are some of the stories of compassion told by people touched by cancer.
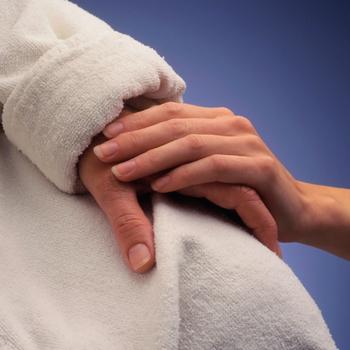
Screening cancer caregivers for symptoms of depression could alert them to steps to offset the worsening physical health which caregivers often experience.

Published: August 3rd 2017 | Updated:

Published: August 10th 2017 | Updated:
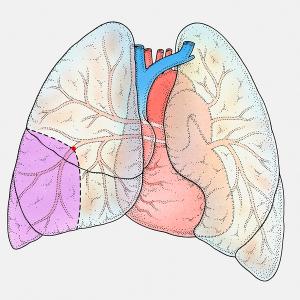
Published: August 11th 2017 | Updated:
Published: August 19th 2017 | Updated:

Published: October 5th 2017 | Updated: