Articles by Angelica Welch

The FDA has granted a priority review designation to a supplemental new drug application (sNDA) for Lynparza (olaparib) tablets for use as a maintenance therapy in patients with newly-diagnosed, BRCA-positive advanced ovarian cancer who achieved a complete or partial response to standard frontline platinum-based chemotherapy.

Three CDK4/6 inhibitors have been approved, offering new options to those with metastatic, hormone-driven breast cancer.
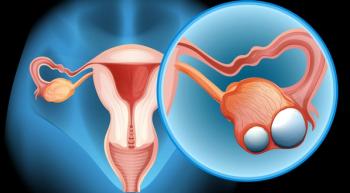
The PARP inhibitor Lynparza (olaparib) significantly improved progression-free survival (PFS) as frontline maintenance therapy for women with BRCA-positive advanced ovarian cancer, according to findings from the randomized phase 3 SOLO-1 trial presented at the 2018 ESMO Congress.

Some patients eligible for bladder surgery due to cancer may safely opt to preserve the organ.

After chemotherapy, immunotherapy has become the standard treatment for bladder cancer. Will it ever play a bigger role?

An immunotherapy pair looks promising for those with pleural mesothelioma, which is now treated with chemotherapy.

There were monumental advances in the treatment of bladder cancer during the year 2017, with five approvals for checkpoint inhibitors in both the first and second line setting
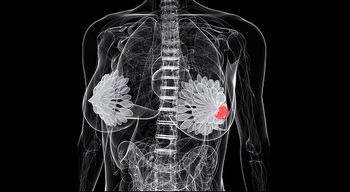
Treatment options for patients with HER2-positive breast cancer continue to expand, especially after the Food and Drug Administration (FDA) approval of Nerlynx (neratinib) earlier in 2017 and the more recent priority review designation of Perjeta (pertuzumab).
A researcher discusses the exciting times surrounding the development of novel agents designed to treat multiple myeloma.
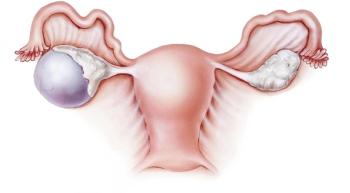
Checkpoint inhibitors in combination with PARP inhibitors are garnering interest, with the impact of PARP inhibitors already well established in this tumor type.

Marina C. Garassino, M.D., talks about exciting advances in the field of lung cancer.
Clinical trials of combination immunotherapy treatments are showing impressive overall survival rates.

More patients with non-small cell lung cancer (NSCLC) should be evaluated and referred to clinical trials, especially those who may have molecular abnormalities, says Victoria M. Villaflor, M.D.

The way that newly diagnosed patients with multiple myeloma is treated has changed significantly over recent years, especially as new drugs moved into the treatment realm for the disease, says Shaji K. Kumar, M.D.

A number of patients with lung cancer have benefitted from immunotherapy agents, and researchers in the field are excited that these treatments will continue to improve the field.

In an interview with CURE, Ruben Mesa, M.D., director, UT Health San Antonio Cancer Center, shed light on these recent updates in PV and ET, and discussed the proper therapeutic management of these diseases.

A more personalized approach is needed in treatment plans for patients with stage 3 non-small cell lung cancer (NSCLC), according to Thomas A. Hensing, M.D.

Merrick I. Ross, M.D., discussed key issues in melanoma as the treatment paradigm continues to change.

“It is not so much that the biology is changing; it’s that our knowledge of the biology is improving,” said Brant Inman, M.D.
A phase 2 trial – which is still enrolling patients – is investigating multiple targeted agents for the treatment of patients with glioblastoma (GBM).
Up-and-coming treatments for patients with triple-negative breast cancer (TNBC) may include immunotherapy combinations, according to Sylvia Adams, M.D.
In a recent study, researchers were working on identifying different molecular targets to better provide personalized care to patients with ovarian cancer. To do this, they examined more than 4,000 tumor specimins.

We’re moving into a new era of understanding with genetic abnormalities in colorectal cancer (CRC) as researchers continue to investigate tumor sidedness and the role of microsatellite instability (MSI) testing.
Eunice Wang, M.D., discusses a promising new drug for the treatment of certain patients with acute myeloid leukemia.
Obesity can negatively impact breast cancer outcomes, and an ongoing study is looking into why -- and how these risks can be mitigated.

Edward Messing, M.D., discusses his recent paper which explored a possible way to reduce recurrence in some patients with bladder cancer.
There is currently no uniform procedure for patients with double-hit lymphoma (DHL) that prolongs benefit. Some patients receive autologous stem-cell transplantation to reduce the risk of relapse, but the jury is still out on the impact that it has.
There are 16 known genes that cause kidney cancer and at least 13 different types of inherited kidney cancer, emphasizing the need to understand genetic differences between these disease.
In an interview with CURE, Katz, professor of Medicine and Health Management and policy at the University of Michigan, discussed his study of patient reaction to surgeon recommendations about CPM.


































